Transition metal dichalcogenide quantum dots based optical detection platform for Cu2+ ions in water
Main Article Content
Article Sidebar
G. B. V. S. Lakshmi
Pratima R. Solanki Kedar Singh
Abstract
Heavy metal ions pollution is one of the most serious concerns in the 21st century for human health and environment due to the drastic growth of industrial revolutions as well as population growth. Heavy metal ions are the non-biodegradable substances that accumulate in the human body through car emissions, industrial activities, food chain, and beverages [1]. Copper (Cu), similar to iron (Fe), zinc (Zn), and cobalt (Co), are essential trace metals found in cells and tissues. It is the third most abundant trace element found in the human body. Cu is a transition metal with oxidation states such as Cu (0), Cu (I), and Cu (III) (II) [2]. Cu exists in several salts, including copper chloride, copper oxide, and copper sulfate, among others. Copper-dependent enzymes play an important role in the biological functions of the human body, various micro/macro-organisms, plants and animals. According to the reported literature, Cu intake of 80 to 100 mg in the adult human body is hazardous and causes a range of ailments, according to the literature [3]. Extensive and excessive consumption of Cu leads to damage of DNA strands, irregulating of antioxidant enzyme function, brain damage, and other acute diseases like fever, nausea, headache, vomiting, diarrhoea, etc. The permissible limit of Cu for a healthy person is below 5 mg [4]. Cu is consumed into the human body through drinking water, food products, air-breathing, and skin contact with copper-containing materials [5]. It is important to identify the quality of drinking water and harmful metal ions found in industrial waste. In order to approach metal ions detection, a quick responsive, sensitive and robust analytical method is required.
Transition metal dichalcogenide quantum dots (TMD QDs) such as MoS2QDs, WS2QDs, MoSe2, VS2QDs, etc. are the most promising candidates among conventional techniques for the detection of metal ions [6, 7]. Due to their ease of synthesized, soft handling, and cost-effectiveness [8, 9]. Moreover, QDs having the ultra-small size, band edge, high surface-volume ratio, strong quantum confinement, band edge, leading to the unique physio-chemical, optical, electronic, and electrochemical properties compared to their bulk [7]. Apart from this, QDs can be easily functionalized or capsulate with functional groups/capping groups such as peptides, amino acids, small water-soluble organic molecules including thioglycolic acid (TGA) etc [10]. The other advantage of this capping group is that QDs become more biocompatible as well as affect photophysical processes and bandgap. Herein, we have synthesized TGA@VS2QDs via a facile two-step hydrothermal method. TGA@VTGA acts as the Sulfur source and capping group to making QDs water-soluble [11]. Apart from this, TGA also provides high stability to QDs by preventing the agglomeration of QDs and enhances the biocompatibility properties of QDs [12]. The present study was proposed the interaction of TGA@VS2QDs with environmental pollutant metal ions Zn2+, Pd2+, Fe2+, Cu2+, K+, Na+, Ca+2, and Mg+2 in the aqueous solution through PL spectroscopy. The PL study showed the specific interaction towards Cu2+ using TGA@VS2QDs. The response study was measured in two concentration ranges from 10-8 M – 10-5 M & 10-5 M – 10-3 M and observed the linear decrease in PL intensity. While other metal ions Zn2+, Pd2+, and Fe2+, K+, Na+, Ca+2, and Mg+2 didn’t show sequential quenching with TGA@VS2QDs. The quenching process in case Cu+2 against the TGA@VS2QDs is due to the static quenching phenomenon and it was confirmed by UV-visible and PL studies with limit of detection (LOD) of 50 nM.
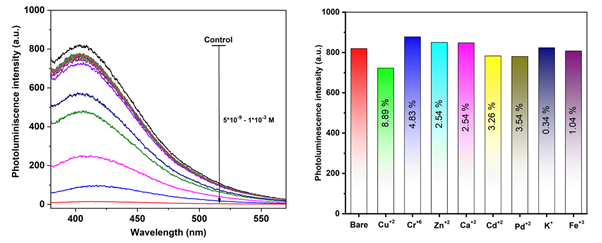
Fig.1 Interaction of TGA@VS2QDs towards Cu+2 ion Fig.2 Inference studied with different metal ions against TGA@VS2QDs (Control)
How to Cite
Article Details
quantum dots, optical, Copper, thioglycolic acid, Transition metal dichalcogenide
2) Chen, Kuo-Yu & Zeng, Wei-Yu. (2021). Adsorption of Cu(II) by Poly-γ-glutamate/Apatite Nanoparticles. Polymers. 13. 962. 10.3390/polym13060962.
3) Trumbo, P & Yates, A.A. & Schlicker, S & Poos, M. (2001). Dietary Reference Intakes - Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Journal of the American Dietetic Association. 101. 294-301. 10.17226/10026.
4) Sadhra, Steven S., Andrew D. Wheatley, and Hilary J. Cross. "Dietary exposure to copper in the European Union and its assessment for EU regulatory risk assessment." Science of the total environment 374.2-3 (2007): 223-234.
5) Pizarro, Fernando, et al. "Gastrointestinal effects associated with soluble and insoluble copper in drinking water." Environmental Health Perspectives 109.9 (2001): 949-952..
6) Cao, Xuanyu, et al. "Transition metal dichalcogenide quantum dots: synthesis, photoluminescence and biological applications." Journal of Materials Chemistry B 6.48 (2018): 8011-8036.
7) Kumar, Rahul, et al. "A novel approach towards optical detection and detoxification of Cr (VI) to Cr (III) using L-Cys-VS2QDs." Journal of Environmental Chemical Engineering 7.4 (2019): 103202.
8) Kumar, Rahul, et al. "Highly sensitive amoxicillin immunosensor based on aqueous vanadium disulphide quantum dots." Journal of Electroanalytical Chemistry 892 (2021): 115266.
9) Hashmi, S. Z. H., Dhiman, T. K., Chaudhary, N., Singh, A. K., Kumar, R., Sharma, J. G., ... & Solanki, P. R. (2021). Levofloxacin Detection Using L-Cysteine Capped MgS Quantum Dots via the Photoinduced Electron Transfer Process. Frontiers in Nanotechnology, 3, 2.
10) Sajwan, R. K., Pandey, S., Kumar, R., Dhiman, T. K., Eremin, S. A., & Solanki, P. (2021). Enhanced fluorescence of mercaptopropionic acid capped zinc sulfide quantum dots with moxifloxacin in food and water samples via reductive photoinduced electron transfer. Environmental Science: Nano.
11) Du, Cuicui & Shang, Anqi & Shang, Mengxiang & Ma, Xiaohan & Song, Wenbo. (2017). Water-soluble VS 2 quantum dots with unusual fluorescence for biosensing. Sensors and Actuators B: Chemical. 255. 10.1016/j.snb.2017.08.070.
12) Rosenthal, Sandra & Chang, Jerry & Kovtun, Oleg & McBride, James & Tomlinson, Ian. (2011). Biocompatible Quantum Dots for Biological Applications. Chemistry & biology. 18. 10-24. 10.1016/j.chembiol.2010.11.013.

 https://orcid.org/0000-0003-0006-7267
https://orcid.org/0000-0003-0006-7267