CeO2/TiO2 based nano composite for photocatalytic degradation of Azo-dyes: Nitrophenol and Phenol red
Main Article Content
Article Sidebar
TARUN KUMAR DHIMAN
Pawan S. Rana Pratima R. Solanki
Abstract
Toxic pollutants from several industries have been the severe threat to the environment. The most common and harmful pollutants are the organic pollutants. Among which persistent organic pollutants (POPs) create a concern because of their and bioaccumulation in animals (1) long range transport ability, toxicity, and persistence. (2) Due to these characteristics POPs travel long distance to pollute the water bodies and are preserved in the living beings. Among these some are carbon based toxic compounds which are released as the waste materials from industries such as, dibenzofurans, polychlorinated dibenzo-pdioxins and polychlorinated biphenyls, and organochlorine pesticides like dichloro-diphenyl-trichloroethane/ hexachlorobenzene, dibenzo-p-dioxins and dibenzo-p-furans. (3) These organic products are discharged in the environmental surrounding in the form of byproducts of processes like waste cremation/metal production. Few among them have ability to bioaccumulate and bio magnify. There have been several studies going on for eliminating high toxic compounds from the water via methods like ozonation, precipitation, ion exchange, adsorption, advanced oxidation and reverse osmosis etc. but some of the processes have not been feasible because of their high cost. (4) There are several classifications of the dyes such as, acidic dye, basic dye, direct dyes, fluorescent dyes, Sulphur dye, reactive dyes, vat dyes and precursor dyes. Among which we are focusing currently on the acidic dye. Acidic dyes are those which are water soluble. These compounds have carboxylic acid groups/sulphonic in the molecules. Chemical constitution of these compounds is azo, triarylmethanes and anthraquinones, nitro, iminoacetone, quinoline and nitrous. The azo dyes are characterized by the different azo group (-N=N-) present in different number. These are linked with the phenyl and naphthyl radicals, these are further replaced by functional groups such as chlorine, amino, hydroxyl, nitro, methyl, sodium salts and sulphonic acid. (5,6) Due to the above mention functional groups azo dyes are considered as mutagenic and carcinogenic. (7,8,9) Several research and studies have shown that release of such dyes into environment alarming because of their being toxic damages to exposed organisms. There have been studies going on degradation of organic dyes via
Physical processes such as flocculation, adsorption, silica gel, membrane filtration, activated coal, UV radiation, wood chips, ion exchange technology, coagulation by electro kinetics, filtration process. Chemical processes are also used, these includes oxidation, Fenton reaction, ozonation, photochemical processes etc. (10,11) Azo dyes are barely biodegradable because of their extreme stability towards light and resistance towards microbial attack. They are also resistant to natural biodegradation. (12) Organic compound with phenolic group and its derivatives is the commonly found pollutant which are released from the industries. Among which nitrophenol is the most toxic. It is generated from the former explosives or fabric factories and military plants and contaminate the soil and water bodies. The reduction of Nitrophenol and other nitroaromatics is very crucial, due to their anthropogenic, toxic and inhibitory characteristic. Current research is aimed at remediation of Nitrophenol and phenol red polluted water with the use of CeO2/TiO2 Nanomaterial composite via photocatalysis process. (13) Here we have studied photocatalytic degradation of Nitrophenol (NP), and phenol red (PR). We have studied the two azo dyes, (NP and PR) without light under dark, with visible light (λ>400nm), and with UV light (λ>400nm). Fig. 1(a), (b) and (c) shows UV–vis absorption spectra of catalysis and photocatalysis performed by CeO2/TiO2 on NP without light under dark, with visible, and with UV light. Fig. 1 (a) i.e., without light under dark. and (b) i.e., with visible light, exhibit no notable change in the UV absorption spectra. Fig. 2(c) with UV light, a substantial decrease could be observed in UV absorbance spectra. Fig. 2(a), (b), and (c) shows UV–vis absorption spectra of catalysis and photocatalysis performed by CeO2/TiO2 on PR without light under dark, with visible light, and with UV light. Similar trend was observed for PR as in NP. The max degradation percentage for NP was 97% and for PR was 99% obtained. The reaction rate constant (k) was 0.42 for NP and 0.54 for PR.
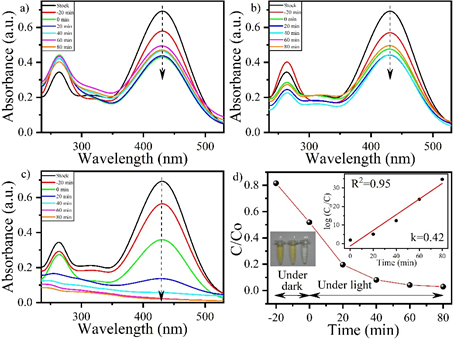
Fig. 1. UV–vis spectra for NP (a) without light, (b) with visible light (λ>400nm), (c) with UV light (λ<400nm), (d) shows drop in the intensity of NP with time. Inset exhibit the kinetic study based on pseudo-first-order for degradation studied under UV (plot (c)), and image exhibit the change of dye color for (i) stock solution, (ii) post 20 min UV irradiation, and (iii) post 60 min UV irradiation.
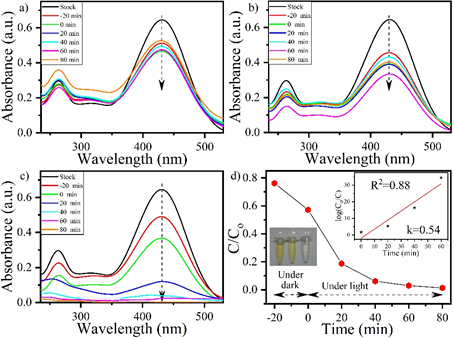
Fig. 2. UV–vis spectra for PR (a) without light, (b) with visible light (λ>400nm), (c) with UV light (λ<400nm), (d) shows drop in the intensity of NP with time. Inset exhibit the kinetic study based on pseudo-first-order for degradation studied under UV (plot (c)), and image exhibit the change of dye color for (i) stock solution, (ii) post 20 min UV irradiation, and (iii) post 60 min UV irradiation.
How to Cite
Article Details
p-Nitrophenol, Phenol Red, TiO2, CeO2, Photocatalysis, Nanocomposite
2. Harrad,. (2001). Persistent Organic Pollutants Enviromental Behaivour and Pathways for Human Exposure. 10.1007/978-1-4615-1571-5.
3. Rashed, Mohamed Nageeb. (2013). Adsorption technique for the removal of organic pollutants from water and wastewater. Organic Pollutants-Monitoring, Risk and Treatment. 167-194.
4. Ventura-Camargo, Bruna & Marin-Morales, Maria. (2013). Azo Dyes: Characterization and Toxicity– A Review. Textiles and Light Industrial Science and Technology. 2.
5. Idaka, Eiichi & Ogawa, Toshihiko & Horitsu, Hiroyuki. (1987). Reductive metabolism of aminoazobenzenes by Pseudomonas cepacia. Bulletin of environmental contamination and toxicology. 39. 100-7. 10.1007/BF01691796.
6. Robinson, Tim & Mcmullan, Geoff & Marchant, Roger & Nigam, Poonam. (2001). Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource technology. 77. 247-55. 10.1016/S0960-8524(00)00080-8.
7. Jadhav, Jyoti & Parshetti, G.K. & Kalme, S.D. & Govindwar, Sanjay. (2007). Decolourization of azo dye methyl red by Saccharomyces cerevisiae MTCC 463. Chemosphere. 68. 394-400. 10.1016/j.chemosphere.2006.12.087.
8. Dhiman, Tarun K., and Satyendra Singh. "Enhanced Catalytic and Photocatalytic Degradation of Organic Pollutant Rhodamine‐B by LaMnO3 Nanoparticles Synthesized by Non‐Aqueous Sol‐Gel Route." physica status solidi (a) 216.11 (2019): 1900012.
9. Ahlawat, Amit & Rana, Pawan & Solanki, Partima. (2021). Studies of photocatalytic and optoelectronic properties of microwave synthesized and polyethyleneimine stabilized carbon quantum dots. Materials Letters. 130830. 10.1016/j.matlet.2021.130830.
10. Kujur, Vidya & Singh, Satyendra. (2020). Structural, magnetic, optical and photocatalytic properties of GaFeO3 nanoparticles synthesized via non-aqueous solvent-based sol–gel route. Journal of Materials Science: Materials in Electronics. 27. 1-14. 10.1007/s10854-020-04318-2.

 https://orcid.org/0000-0001-7602-1068
https://orcid.org/0000-0001-7602-1068