Degradation of Methyl Parathion using Manganese oxide (MnO2) nanoparticles through photocatalysis
Main Article Content
Article Sidebar
Amit Ahlawat
Tarun Kumar Dhiman
G.B.V.S. Lakshmi
Pratima R. Solanki
Abstract
Methyl parathion is a type of organophosphate (OP) compound that has been considered a pesticide and warfare agent. This is irreversible enzyme acetylcholinesterase inhibitors causing damage to the nervous system of exposed organisms (respiratory paralysis) and death. [1,2] OP is an ester of phosphoric acid and exists in two forms, ‘Thion and Oxon’. Parathion is a methyl part which is a highly toxic group in nature and broadly used in crops such as mainly cotton, corn, wheat, alfalfa, soybeans, fruits, and vegetables. Domestic work also leads to various hazards. [3,4] Methyl parathion (MP) is known to be O, O‐dimethyl‐O‐4‐p‐nitrophenyl phosphorothioate and it is classified as a class I insecticides. U.S. Environmental Protection Agency has categorized the Methyl parathion as restricted use of pesticides due to its high toxicity level. [5] MP is known as a cotton poison because it is utilized on the large scale in sunflower, and peaches etc. MP is also known as metaphos, is exposed to the environment in the form of agricultural insecticides through spraying using ground spray equipment. [6,7]
MP is harmful to human health and its absorption, inhalation and contact with materials contamination are also responsible for MP exposure. [8,9] It leads to several health issues such as mutagenic effects also influence the DNA sequences. Exposure of MP is also reported for the cardiovascular effects on the organisms in previous studies.[10] MnO2 is the most stable and significant nanomaterial which attracts several researchers for its application. Due to its less toxicity and cost-effectiveness properties, it is considered an ideal nanomaterial and used as a catalyst. [11] MnO2 nps is very promising nanomaterial which is explored in various sectors such as sensing, drug delivery system, rechargeable battery and catalysts etc. [12] Despite having several properties, MnO2 nps is less explored in terms of photocatalytic degradation. Herein, a photocatalytic degradation of MP is reported using MnO2 nps through visible and UV light. [13,14] The MnO2 nps was synthesized using the co-precipitation method and calcined at 350 °C. UV-Visible absorption study was performed to calculate the band gap which was found to be 2.77 eV using Tauc plot. The photocatalytic study was performed without light source, under visible light source, and under UV light source and their corresponding percentage were recorded 40%, 58% and 72%, respectively. The pseudo-first order rate constant (k) was 0.02. [15]
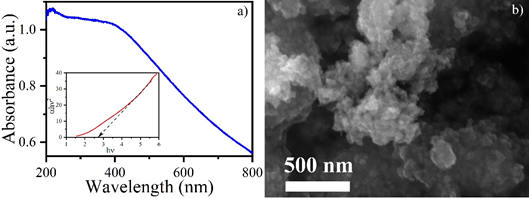
Fig. 1: a) UV-vis absorption spectra of MnO2nps and inset shows the corresponding bandgap, and b) SEM image of the MnO2nps.
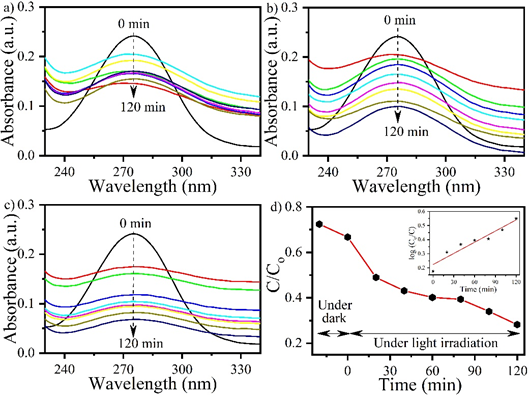
Fig. 2. UV–Visible absorbance plot of MP in presence of MnO2nps performed (a) without light source, (b) under visible light source, (c) under UV light source, and (d) shows the change in the intensity of MP with time. The inset shows the kinetic study of degradation studied under UV light source (plot (c)).
How to Cite
Article Details
Methyl parathion, MnO2, Photocatalysis, Nanoparticles, Pesticides
[2] Cabello, G.; Valenzuela, M.; Vilaza, A.; Duran, V.; Rudolph, I.; Hrepic, N.; Calaf, G.: A rat mammary tumor model induced by the organophosphorus pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environmental Health Perspectives, 2001, 109(5):471-479.
[3] Garimella, L. B., Dhiman, T. K., Kumar, R., Singh, A. K., & Solanki, P. R. (2020). One-step synthesized ZnO np-based optical sensors for detection of aldicarb via a photoinduced electron transfer route. ACS omega, 5(6), 2552-2560.
[4] L. G. Costa, “Organophosphorus compounds,” in Recent Advances in Nervous System Toxicology, C. L. Galli, L. Manzo, and P. S. Spencer, Eds., vol. 100 of NATO ASI Series, pp. 203– 246, Plenum Press, New York, NY, USA, 1988.
[5] U.S. Environmental Protection Agency. Chemical Emergency Preparedness and Prevention: EPA Chemical Profile. Revised 30 November 1987.
[6] Ruckart, P. Z.; Kakolewski, K.; Bove, F. J.; Kaye, W. E.: Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environmental Health Perspectives, 2004; 112(1):46-51.
[7] United State Geological Survey. 1997 Pesticide use maps: Methyl parathion-insecticides estimated annual agricultural use. Updated 13 August 2003.
[8] Garcia, S. J.; Abu-Qare, A. W.; Meeker-O’Connel, W. A.; Borton, A. J.; Abou-Donia, MB. Methyl parathion: A review of health effects. Journal of Toxicology and Environmental Health, 2003b; 6:285-210.
[9] Zhu, H.; Rockhold, R. W.; Baker, R. E.; Kramer, R. E.: Effects of single or repeated dermal exposure to methyl parathion on behavior and blood cholinesterase activity in rats. Journal of Biomedical Science, 2001; 8:467-474.
[10] Environmental Toxicology and Health Effects Associated with Methyl Parathion Exposure – A Scientific Review.
[11] Singh, A. K., Dhiman, T. K., Lakshmi, G. B. V. S., & Solanki, P. R. (2021). Dimanganese trioxide (Mn2O3) based label-free electrochemical biosensor for detection of Aflatoxin-B1. Bioelectrochemistry, 137, 107684.
[12] Singh, A. K., Lakshmi, G. B. V. S., Dhiman, T. K., Kaushik, A., & Solanki, P. R. (2021). Bio-Active Free Direct Optical Sensing of Aflatoxin B1 and Ochratoxin A Using a Manganese Oxide Nano-System. Front. Nanotechnol. 2: 621681. doi: 10.3389/fnano.
[13] Dhiman, Tarun K., and Satyendra Singh. "Enhanced Catalytic and Photocatalytic Degradation of Organic Pollutant Rhodamine‐B by LaMnO3 Nanoparticles Synthesized by Non‐Aqueous Sol‐Gel Route." physica status solidi (a) 216.11 (2019): 1900012.
[14] Ahlawat, Amit & Rana, Pawan & Solanki, Partima. (2021). Studies of photocatalytic and optoelectronic properties of microwave synthesized and polyethyleneimine stabilized carbon quantum dots. Materials Letters. 130830. 10.1016/j.matlet.2021.130830.
[15] Kujur, Vidya & Singh, Satyendra. (2020). Structural, magnetic, optical and photocatalytic properties of GaFeO3 nanoparticles synthesized via non-aqueous solvent-based sol–gel route. Journal of Materials Science: Materials in Electronics. 27. 1-14. 10.1007/s10854-020-04318-2.

 https://orcid.org/0000-0003-3626-1643
https://orcid.org/0000-0003-3626-1643