The contribution of polyaniline and gold nanoparticles for enhancing current signals of a streptavidin based immunosensor
Main Article Content
Article Sidebar
Abstract
It is well known that one of the required performances for electrochemical biosensors is high sensitivity. The initial current response of an electrochemical sensor plays an important role to improve its sensitivity [1]. Streptavidin has been commonly used for capturing with biotin-labeled on biorecognition molecule in the process of electrochemical immunosensor fabrication. However, the presence of streptavidin was found to serve as an electrically insulating layer. This could reduce the current response of an electrochemical immunosensor leading to reducing its performance. Therefore, the selection of suitable supporting materials for streptavidin modification is important to develop the immunosensor with high sensitivity for target sample detection in future work. Polyaniline is a conducting polymer and consisting of amine groups that are useful for biomolecule immobilization. In addition, gold nanoparticles have been obtained high attention for enhancing the sensitivity of immunosensor [2, 3]. This work aims to investigate the current response of the streptavidin-based electrochemical immunosensor when modifying the electrode surface using polyaniline and gold nanoparticles for the development of immunosensors.
In this study, polyaniline was used as supporting material to modify the surface of a screen-printed gold electrode for pegging streptavidin via EDC/NHS linker on the surface. Firstly, polyaniline was modified on the working electrode surface of a screen-printed electrode (SPAuE) by electropolymerization process of aniline via cyclic voltammetry. Gold nanoparticles were then electrochemically deposited on polyaniline modified electrodes to maintain the proton doped state of polyaniline, and further, enhance the conductivity of the immunosensor. After that streptavidin was bound on the modified electrode via EDC/NHS as a cross-linker. Each step of the sensor modification was investigated using differential pulse voltammetry (DPV) which was performed in 10 mM K4[Fe(CN)6]/K3[Fe(CN)6] containing 10mM phosphate buffer, pH 7.0 as shown in Fig 1.
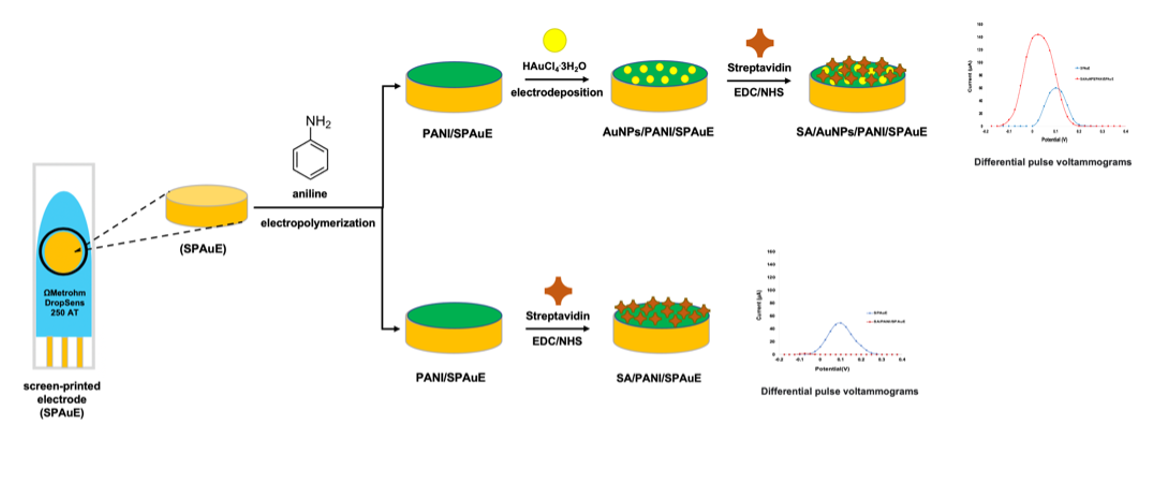
Fig. 1 Schematic illustration of the different methods of streptavidin-based modification on a screen-printed gold electrode
The result revealed that the conductivity of the modified electrode increased noticeably when streptavidin was bound on the polyaniline layer with the presence of gold nanoparticles (Fig 2A red line). As mentioned above, there have been reported that streptavidin exhibited as insulating material when was bound on sensor surface [5,6]. However, it is noticed that the current signal of the sensor increased when streptavidin was immobilized. This can explain that streptavidin becomes a conductive material by influencing the dual amplified signal of both polyaniline and gold nanoparticles that induce some of the amino-acid residue streptavidin as electroactive [4], and facility electron transfer through streptavidin [5]. In contrast, the streptavidin-modified polyaniline electrode surface without gold nanoparticles exhibited a significant decrease in the current signal as illustrated in Fig. 2B (red line), which is consistent with the previous study [6]. Moreover, there was a previous report about streptavidin modified with gold nanoparticles without polyaniline that showed non-conductivity as well. Hence, the result could suggest that the high conductivity of streptavidin is derived from the contribution of both polyaniline and gold nanoparticles.
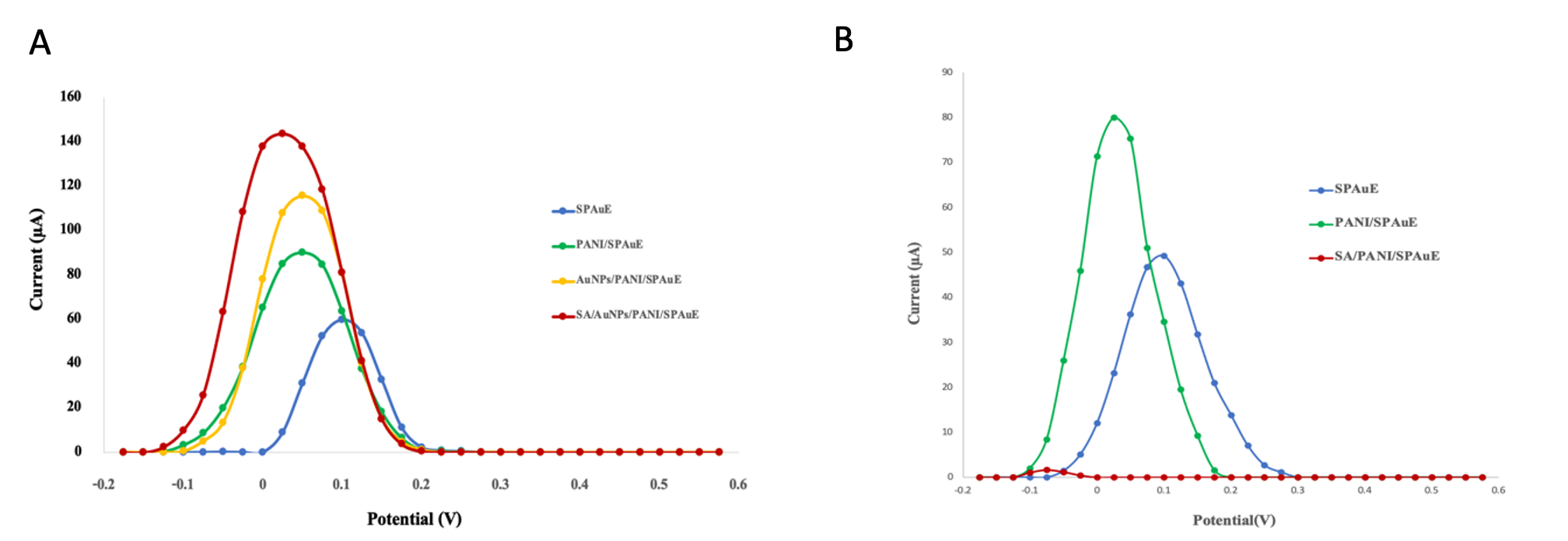
Fig.2. Differential pulse voltammograms show (A) the resulting current response at streptavidin/AuNPs/PANI on screen-printed electrodes (SPAuE), and (B) at streptavidin/PANI/SPAuE (without AuNPs).
As mentioned above, there have been reported that when streptavidin was bound on a sensor surface, the current signal reduced since the streptavidin exhibited as insulating material [5,6]. Interestingly, the result revealed that the current response of the modified electrode increased noticeably when streptavidin was bound on the polyaniline layer with the presence of gold nanoparticles (Fig 2A red line). This can explain that streptavidin becomes a conductive material by influencing the dual amplified signal of both polyaniline and gold nanoparticles that induce some of the amino-acid residue streptavidin as electroactive [4], and facility electron transfer through streptavidin [5].In contrast, the streptavidin-modified polyaniline electrode surface without gold nanoparticles exhibited a significant decrease in the current signal as illustrated in Fig. 2B (red line), which is consistent with the previous study [6]. Moreover, there was a previous report about streptavidin modified with gold nanoparticles without polyaniline that showed non-conductivity as well. Hence, the result could suggest that the high conductivity of streptavidin is derived from the contribution of both polyaniline and gold nanoparticles.
The use of both polyaniline and gold nanoparticles could support the streptavidin modified immunosensor leading to enhancing multifold sensitivity of immunosensor. The dual integration of both polyaniline and gold nanoparticles provides a powerful method to improve the performance of a streptavidin-modified sensor for use in immunosensor development.
How to Cite
Article Details
Polyaniline, Gold nanoparticles, Immunosensor, streptavidin, Electrochemistry
[1] X. MA, Y. Jiang et al. Journal of microbiological methods, 98, 94-98(2014).
https://doi.org/10.1016/j.mimet.2014.01.003
[2] K. Arshak, V. Velusamy, et al. IEEE Sensors journal, 9(12), 1942-1951(2009).
https://doi.org/10.1109/JSEN.2009.2032052
[3] J. Wang, L. Wang, J. Di, Y. Tu, Talanta, 77(4), 1454-1459 (2009).
https://doi.org/10.1016/j.talanta.2008.09.034
[4] B. Zhang, W. Wang et al. Journal of the American Chemical Society, 142(13), 6432-6438(2020).
https://doi.org/10.1021/jacs.0c01805
[5] Y. Peng, J. Jiang, R. Yu, Analytical Methods, 6(9), 2889-2893 (2014).
https://doi.org/10.1039/C4AY00033A
[6] C. Hu, W. Dou, G. Zhao, Electrochimica Acta, 117, 239-245 (2014).
https://doi.org/10.1016/j.electacta.2013.11.132
