Synthesis of Aromatic Hyperbranched Polymer based on Diphenolic Acid and Pentaerythritol: Reaction Kinetics using FTIR technique
Main Article Content
Article Sidebar
Abstract
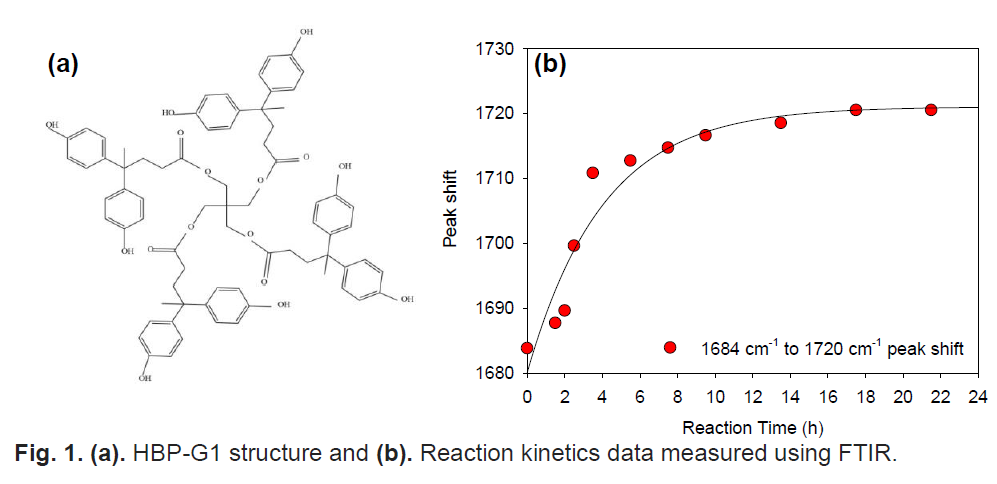
Hyper branched polymers (HBPs) are an important class of materials which have generated much interest among researchers owing to their unique properties such as highly-branched architecture, diverse functionalities and easier synthetic processes with applications ranging from biomaterials, nanocomposites, optoelectronics, polymer coatings, to name a few [1,2]. However, the challenge is to synthesis the HBPs with well-defined architecture using readily available monomers. In general, HBPs can be polymerized by one-pot process from ABn type monomers, A2 + Bn (n ≥ 3) type monomer pairs, and so on [1]. In this study, we report the sequential melt-polycondensation method synthesis of aromatic HBP. Fig. 1(a) shows the aromatic HBP based on diphenolic acid as monomer (AB2 type) and pentaerythritol (A4 type) as core molecule using para-toluene sulphonic acid as the catalyst in the presence of DMSO solvent. Fig. 1(b) shows the reaction kinetics of the HBP-Gen 1 synthesis using FTIR analysis of samples withdrawn at regular intervals of time. The shift in FTIR peak corresponding to acid from 1684 cm-1 to ester peak at 1720 cm-1 confirms the formation of HBP and it could be observed that maximum conversion was achieved at around 8 h reaction time. Acknowledgement: This work was supported by CSIR, Government of India under CSIR-EMR-II scheme (03(1450)18/EMR-II dt.05-06-2018).
How to Cite
Article Details
Hyperbranched Polymers, Melt-condensation, Diphenolic acid, Pentaerythritol, Reaction kinetics, FTIR
https://doi.org/10.1039/C4TA04841E
[2] X. Li, X. Liu, D. Shi, W. Wei, M. Chen, X. Liu, ACS Macro Lett. 7, 778−782 (2018). https://doi.org/10.1021/acsmacrolett.8b00443
