Characterization and photocatalytic activity of chromium doped Tungsten trioxide for the degradation of toxic dyes
Main Article Content
Article Sidebar
Abstract
Scarcity of pure water becomes a major problem for the living beings on the earth. Various industries like paper, textile, leather and printing etc. disseminates dyes into the water bodies which becomes the main reason for the water pollution, therefore to treat these dyes efficiently becomes a major challenge for the researchers [1]. various methods are being used for degradation of these toxic dyes such as adsorption, bio-treatment, incineration, and adsorption processes but they have disadvantages such as they have toxic and non biodegradable by-product. Now a day’s semiconductor photocatalysis is one of the most effective techniques to degrade the wide range of the contaminants without producing the toxic by-products along with excellent degradation efficiency. Metal oxides got special attention because they exhibit multifunctional behaviour. Tungsten trioxide bestowed with many properties such as optimum band gap, easy mode of formation, low photo-corrosion and physically and chemically stable which are essential requirement in the field of photocatalysis. Due to low band gap of tungsten trioxide, its rate of recombination increases which leads to decreases its efficiency. Researchers are trying to find appropriate strategies to reduce the rate of recombination of electron-hole pairs such as by adding some dopants, by morphological control i.e., by changing their shapes and size, etc. and by heterostructuring with other [2]. Doping with transition metal results in induction of shallow energy levels between valence and conduction band which reduces the rate of recombination of photogenerated electrons and holes produce in redox reactions. Present work is focused on the synthesis and characterization of chromium doped tungsten trioxide and to study its photocatalytic activity [3]. Different doping concentration of chromium (1%, 3%, 5% and 10%) in tungsten trioxide was studied. The synthesized material was characterized by X-Ray Diffractometer (XRD), Fourier Transform Infrared spectroscopy (FTIR), UV-Vis spectroscopy and Scanning electron microscopy (SEM). XRD graphs confirms the formation of pure and chromium doped tungsten trioxide and well matched with the JCPDS No. 83-0951. Various parameter like crystallite size, dislocation density, lattice parameter and interplanar spacing were also calculated with the help of XRD. Absence of any extra peak confirms that no impurities is present in the synthesized sample. The synthesis of Cr doped WO3 is also confirmed by FTIR. Morphology of the synthesized material was analyzed by SEM images and comes out to be spherical. The band gap of pristine and Cr doped WO3 were find out by using UV- Vis spectroscopy and comes out to be in visible range. Photocatalytic activity of pristine and Chromium doped Tungsten trioxide were studied towards the cationic dye like methyl violet and anionic dye like cresol red dye. The maximum degradation efficiency for methyl violet and cresol red came out to be 90% and 85% respectively. Therefore, the optimum doping of chromium in tungsten trioxide makes it an efficient and novel material in the field of photocatalysis application
A. B.
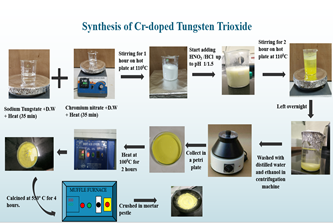
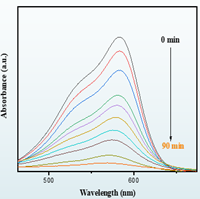
Fig.1. Initial Experiments and results: A. synthesis route of Chromium doped tungsten trioxide B. photocatalytic activity toward Methyl violet dye.
How to Cite
Article Details
photocatalysis, tungsten trioxide, XRD, Dye degradation, methyl violet, cresol red
doi: 10.1016/j.electacta.2018.07.050.
[2] G. Jeevitha, R. Abhinayaa, D. Mangalaraj, and N. Ponpandian, J. Phys. Chem. Solids,
116, 137-147,2018 .
doi: 10.1016/j.jpcs.2018.01.021.
[3] J. Kaur, K. Anand, A. Kaur, and R. C. Singh, Sensors Actuators, B Chem., 258, 1022–
1035, 2018.
doi: 10.1016/j.snb.2017.11.159.
