Poly Neutral Red for Electrocatalytic Hydrogen Evolution Reaction
Main Article Content
Article Sidebar
Abstract
Electrocatalytic hydrogen evolution reaction (HER) is a key process for conversion of renewable electricity to storable hydrogen and is crucial to achieve sustainable energy system. HER currently needs nanoparticles of precious metals as electrocatalysts. Development of alternative catalysts based on abundant materials is therefore necessary. Recently, certain conductive polymers exhibited a high catalytic activity towards HER, as they possess hydrogen bonding sites for stabilization of reaction intermediates [1,2]. Neutral red (NR) is a phenazine dye that has a hydrogen-bonding amino group and can be polymerized as it is an analogue of aniline. We have studied synthesis of poly neutral red (PNR) by oxidative chemical vapor deposition (oCVD) and electropolyerization deposition (EPD) to evaluate their HER electrocatalysis.
F-doped Tin Oxide (FTO, Asahi Glass) and Carbon felt (CF, SIGRATHERM® GFA5) were used as substrates. 3 zone tubular furnace was employed for oCVD in which NR vapor was oxidized by sulfuric acid at 325°C under N2 stream. EPD was performed by potential cycling between -0.2 and 1.2 V (vs. Ag/AgCl) for 50 times in a 5 mM NR - 0.1 M H2SO4 aqueous solution under N2. Polyaniline (PANI) was also obtained by EPD for comparison. The samples were characterized by infrared spectroscopy (IR), and UV-visible spectroscopy (UV/Vis). The HER catalysis was evaluated by linear sweep voltammetry (LSV) in a 1 M trifluoromethanesulfonic acid (TfOH) under N2.
Although both PANI and PNR were nicely obtained by EPD, the CVs during the film growth significantly differed (Fig. 1). An irreversible anodic peak marked as IV is initially seen for oxidation of aniline to trigger its polymerization to PANI. Then, multiple redox peaks marked as I, II, III continue to grow during the CV scans, which are caused by proton-coupled reversible redox of PANI. On the other hand, an irreversible anodic peak II and reversible couple I are seen for NR. While oxidation of NR results in formation of PNR associated with slight decrease of the magnitude of II, the couple I should be proton-coupled reversible reduction of NR, which gradually become irreversible. It already is a sign of catalytic HER by PNR. It is important to note that no redox peaks of PNR are seen as those of PANI.
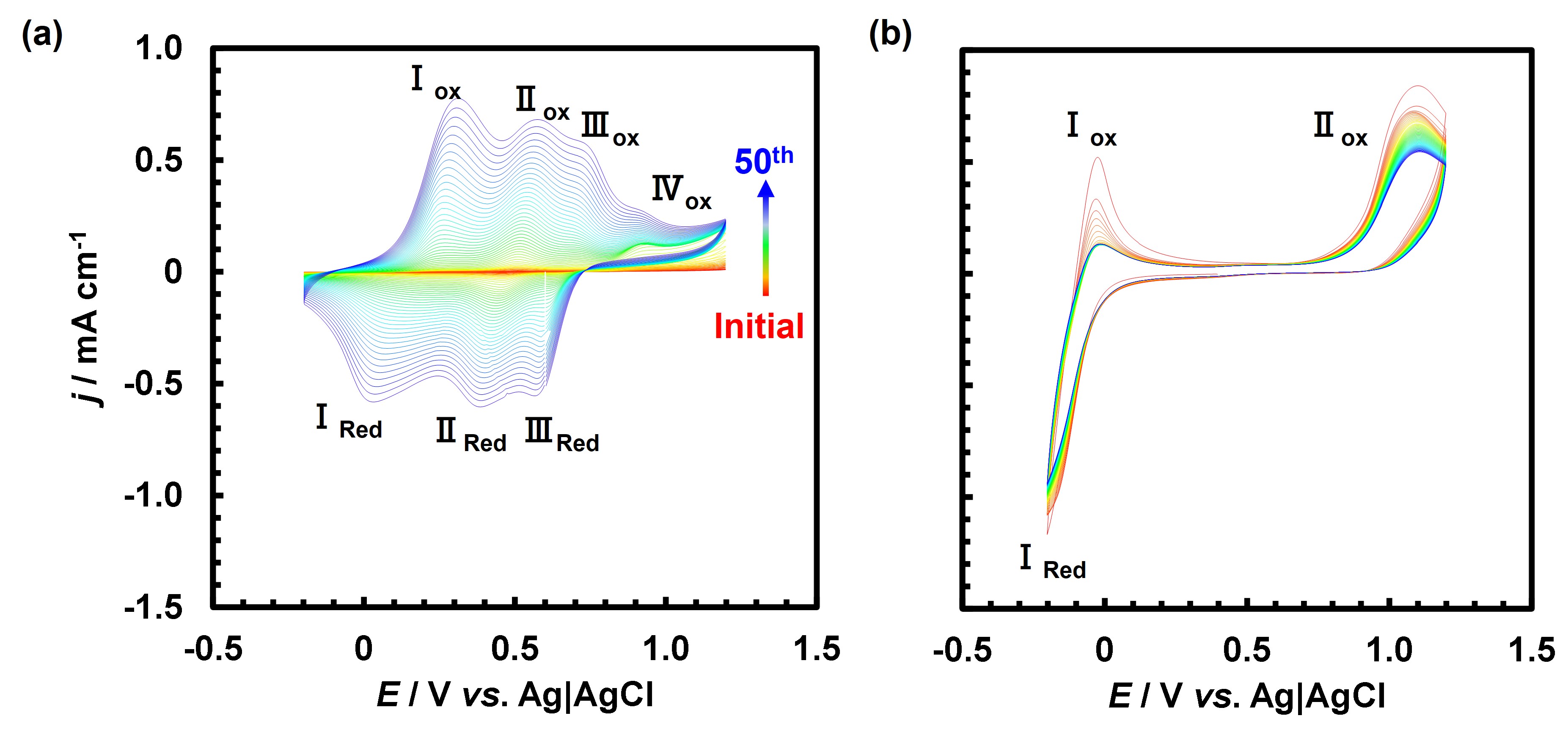
Fig.1. CVs during EPD of (a) PANI and (b) PNR at 50 mV/s in a 5 mM monomer – 0.1 M H2SO4 on FTO for 50 cycles (Red initial to Blue final).
Although significantly broadened, the absorption spectrum of PNR-EPD preserves the character of that of NR monomer, to make it appear dark red (Fig. 2a). On the other hand, PNR-oCVD was black with a featureless spectrum. The FTIR of PNR-EPD also show peaks from the C=C and C=N stretching of the ring system, which are almost in the same positions as those for NR, whereas they are greatly shifted towards shorter wavenumbers for PNR-oCVD, suggesting complete change of the chemical structure during oCVD (Fig. 2b). Both PNR samples, however, show additional peaks assignable to HSO4- or SO42- introduced as dopant to make them conductive.
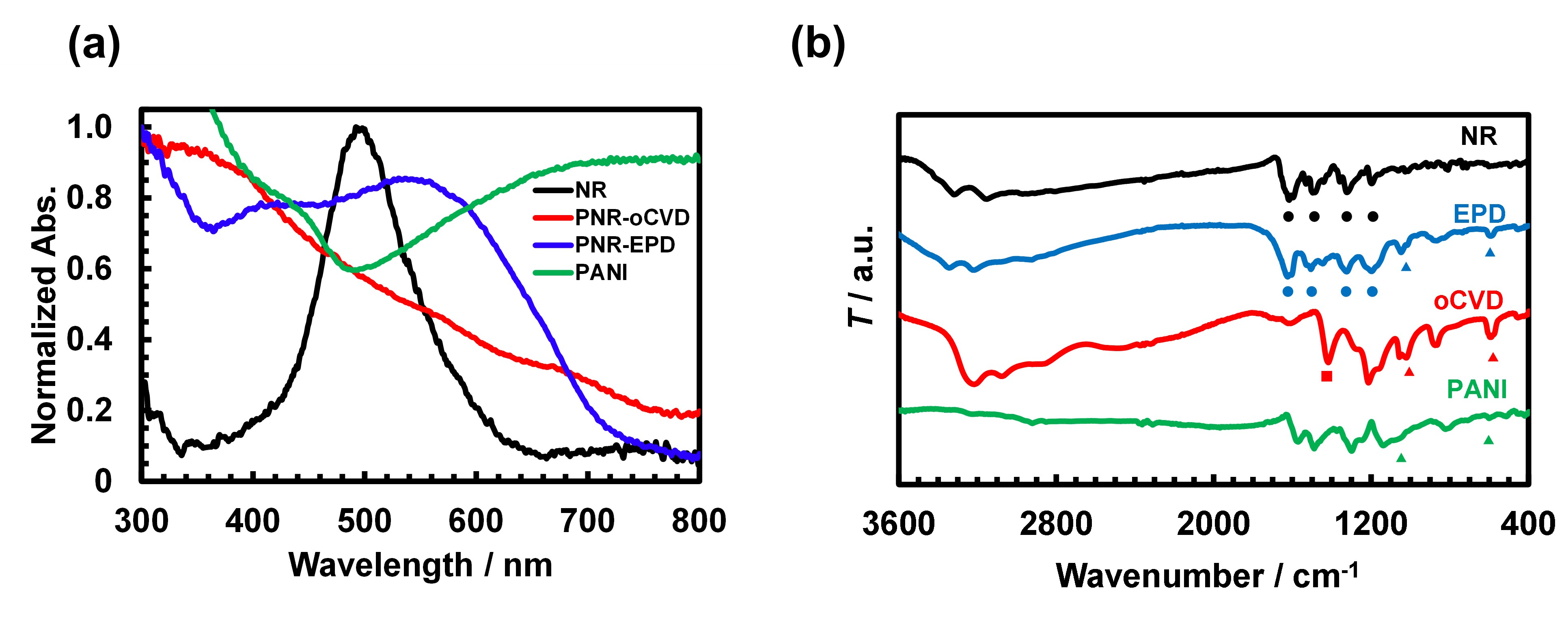
Fig.2. Spectroscopic characterizations: (a) Normalized UV-vis absorption spectra and (b) FTIR spectra of NR, PNR-oCVD, PNR-EPD and PANI. (●: C=C or C=N stretching, ■: δs CH3, ▲: HSO4- / SO42- stretching)
The catalytic activity was in the order of PNR-EPD, PNR-oCVD and PANI with the HER overpotential (η for 10 mA cm-2) of 268, 379 and 590 mV, respectively (Fig. 3a). The best performing PNR-EPD also resulted in the largest exchange current density (i0) and the smallest slope of 0.321 mA cm-2 and 174.2 mV dec-1, respectively, from the Tafel plot (Fig. 3b), which are among the top reported for conductive polymer catalysts [1,2]. The poor HER catalytic activity of PANI is to be mentioned, since high conductivity and proton exchanging capabilities are expected for PANI. Stable reversible redox of PANI as seen in Fig. 1 is an indication of stabilization and localization of additional charge by protonation, which is not irreversibly transferred to proton to yield H2. On the other hand, the extra charge cannot be stabilized in the structure of PNR but rather is transferred to achieve HER. In order for the polymers to be catalytically active, we need to seek for polymers which are conductive as well as hydrogen-bonding, but not redox active.
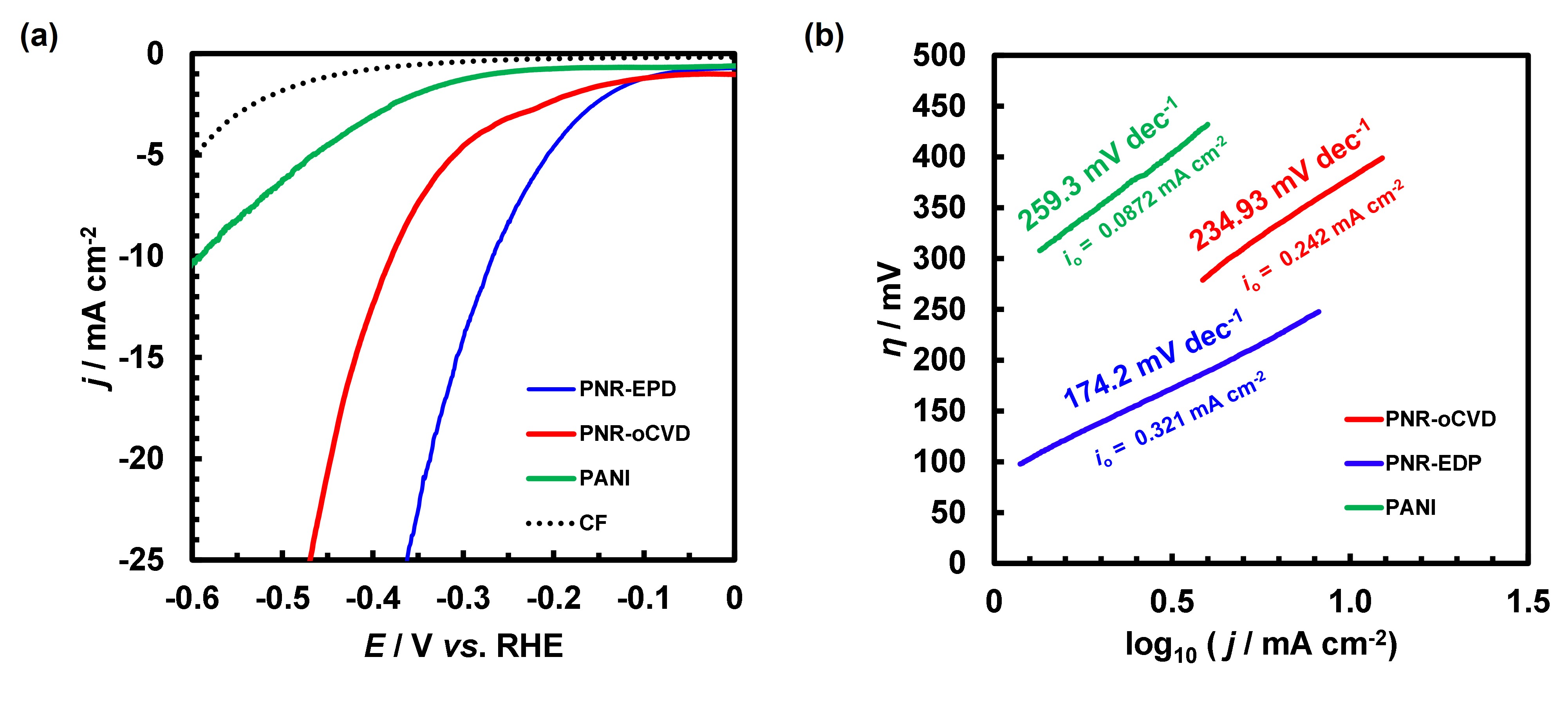
Fig.3. HER catalytic activity evaluation: (a) Liner sweep voltammogram (10 mV s−1) and (b) Tafel plot of PNR-EPD and PNR-oCVD as compared to PANI, CF (blank control) in 1 M TfOH electrolyte.
How to Cite
Article Details
https://doi.org/10.1002/adma.201902177
[2] H. Coskun et al. Advanced Materials Interfaces 7, 1901364 (2020).
https://doi.org/10.1002/admi.201901364
