Electrodeposition synthesis of cobalt-selenide as electrocatalyst for hydrogen evolution reaction
Main Article Content
Article Sidebar
Abstract
The greenhouse effect and air pollution are mainly caused by carbon dioxide which is produced from fossil fuel, coal and natural gas consumption that was used in energy production [1]. Hydrogen energy is one of the alternative energies that has been considered as ideal clean energy and it is also a renewable source [2]. There are many methods to produce hydrogen energy. Among the methods, hydrogen production base on hydrogen evolution reaction (HER) in the electrochemical water splitting is well known as one of the most efficient systems [3]. The significant challenge for water splitting is to develop an active electrocatalyst, that can exhibit high stability, high current density and cost-effective for hydrogen evolution reaction. Generally, platinum is commonly most used as catalyst for the reduction reaction [4, 5]. However, their widespread applications are limited because their scarcity and excessive cost hinder their large-scale commercial applications.
Transition metal chalcogenides (TMCs) are the most promising materials for electrocatalyst due to their adsorption property [2, 6]. However, the TMC catalysts are mostly in powder form. So, the catalyst powders need to be coated on the electrode for its application. This process usually uses binder polymer to attach TMCs on electrode surface but binder polymers such as polytetrafluoroethylene are insulating and electrochemical inert [7]. One possible way to improve the performance of the catalyst is directly growth on the supported electrode.
Herein, the shaggy porous cobalt-selenide (Co-Se) (fig.1(A)) was used instead of platinum, the costly active electrocatalyst. The cobalt-selenide catalyst was directly grown on the synthesized carbon-based porous membrane via the optimized electrodeposition method. The as-prepared shaggy porous cobalt-selenide on carbon porous membrane was directly used as a binder free electrocatalyst for hydrogen evolution reaction. In this work, the porous membrane was first fabricated from cryogenic polymerization of gelatin and glutaraldehyde. Graphene oxide was also doped in the porous membrane to improve its conductive property [8]. After carbonization process, the conductive porous carbon membrane was obtained, and it was directly used as the template for electrodeposition of cobalt-selenide catalyst. The parameters for cyclic voltammetry and chronoamperometry electrodeposition methods were optimized for cobalt selenide synthesis. The concentration of plating solution was optimized for three ratios between Cobalt(II) and selenium(IV). The result showed that 1:1 ratio between Cobalt(II) and selenium(IV) provided the best catalytic efficiency.
The electrochemical properties of electrocatalyst investigation were performed in three electrodes configuration electrolytic cell, including synthesized electrocatalyst, Ag/AgCl in 3.0M KCl, and platinum wire as working electrode, reference electrode, and counter electrode respectively (fig. 1 (B). The performance of hydrogen evolution reaction was performed in acidic (Ar-saturated 0.5 M H2SO4) aqueous electrolytes and linear sweep voltammetry technique was applied. The high electrochemical surface area was observed from the chemical double-layer capacitance (Cdl) with cyclic voltammetry under non-faradaic region. The resistivity of synthesized electrocatalyst are measured from electrochemical impedance spectroscopy technique and it was found that the resistance of Co-Se electrocatalyst from chronoamperometry, cyclic voltammetry and bare electrode equal to 5.99 Ω, 83.36 Ω, 174.32 Ω, respectively. In this project, cobalt selenide electrocatalyst from the optimum condition exhibit overpotentials 116 mV and Tafel slope of 80.1 mV/dec for HER testing. The duration stability of catalysis was performed with chronoamperometry and the results provide no decreasing of cathodic current over 24 hours testing period.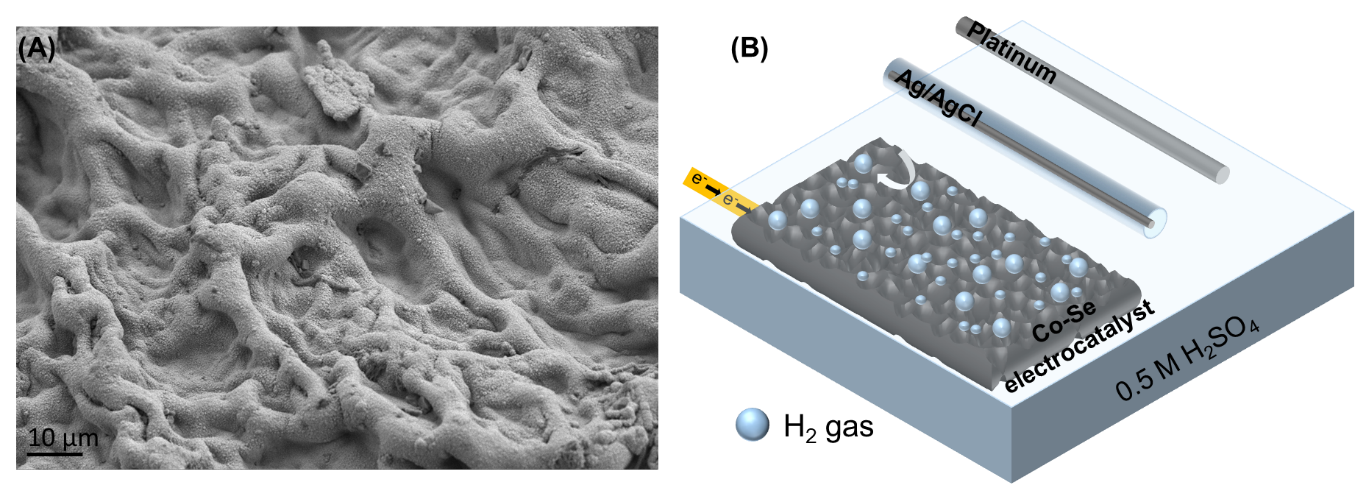
How to Cite
Article Details
[2] Ren, H., et al. Journal of Energy Chemistry. 60: p. 194-201 (2021) DOI: https://doi.org/10.1016/j.jechem.2021.01.002.
[3] Dincer, I. and C. Zamfirescu. International Journal of Hydrogen Energy. 37(21): p. 16266-16286 (2012) DOI: https://doi.org/10.1016/j.ijhydene.2012.02.133.
[4] Naito, T., et al. Inorganic Chemistry Frontiers. 8(11): p. 2900-2917 (2021) DOI: https://doi.org/10.1039/D0QI01465F.
[5] Zhang, Z., et al. RSC Advances. 3: p. 16528 (2013) DOI: https://doi.org/10.1039/C3RA42360C.
[6] Chen, W., et al. ACS Central Science. 1(5): p. 244-251 (2015) DOI: https://doi.org/10.1021/acscentsci.5b00227.
[7] Wang, J., et al. Advanced materials. 29(14): p. 1605838 (2017) DOI: https://doi.org/10.1002/adma.201605838.
[8] Poorahong, S., et al. Journal of Electroanalytical Chemistry. 897: p. 115568 (2021) DOI: https://doi.org/10.1016/j.jelechem.2021.115568.
