AIE–active ferrocene appended linear chromophores: Structural, theoretical and effect of phenyl group on NLO properties
Main Article Content
Article Sidebar
Abstract
The development of new organometallic material with push-pull systems has been utilized in various nonlinear optical applications such as electro-optic modulators, optical data storage devices, telecommunications, optical switches, and NLO bioimaging [1-3]. Herewith, a new and effective D-π-A/ D-π-A-π-A system has been synthesized based on ferrocene core using Knoevenagel condensation reaction, and the chromophores 1-4 were characterized using various analytical and spectroscopic techniques. Further, the chromophores 2 and 4 were structurally confirmed by single‐crystal X‐ray diffraction studies, which shows centrosymmetric space group along with various non-covalent interactions in the crystal packing (Fig. 1). The charge transfer ability of chromophores 1-4 were examined through the solvatochromic technique, which demonstrates positive solvatochromism, because of the high excited-state dipole moment than ground states. The low fluorescence to enhanced fluorescence intensity was achieved by aggregation-induced emission (AIE) studies using the principle of restriction of intramolecular rotations (RIR) in the THF: H2O mixture. The chromophores 1-4, bulk second-order nonlinear optical (NLO) properties were carried out by Kurtz and Perry powder method. Though, the chromophores crystallized in a centrosymmetric crystal system, which shows the SHG signal, owing to the presence of strong electron-withdrawing CN and NO2 group and various non-covalent interactions in the crystal packing (Fig. 1). Chromophores 2 and 4 exhibit enhanced SHG efficiency than 1 and 3 (Table 1), hence the first hyperpolarizabilities (β) of 2 and 4 were measured using Hyper Rayleigh scattering (HRS) technique [µβHRS = 56.5 × 10-30 esu (2); µβHRS = 81.5 × 10-30 esu (4)]. Chromophore 4 shows a higher second-order NLO response than chromophore 2, due to the presence of an additional phenyl group which makes 4 more planar and facilities the effective intramolecular charge transfer (ICT) process between donor to the acceptor. The observed results of optical and nonlinear optical properties using experimental methods were further supported by density functional theory (DFT) and time depended-DFT calculations using the B3LYP/6-31+G** level of theory (Table 1) will be presented.
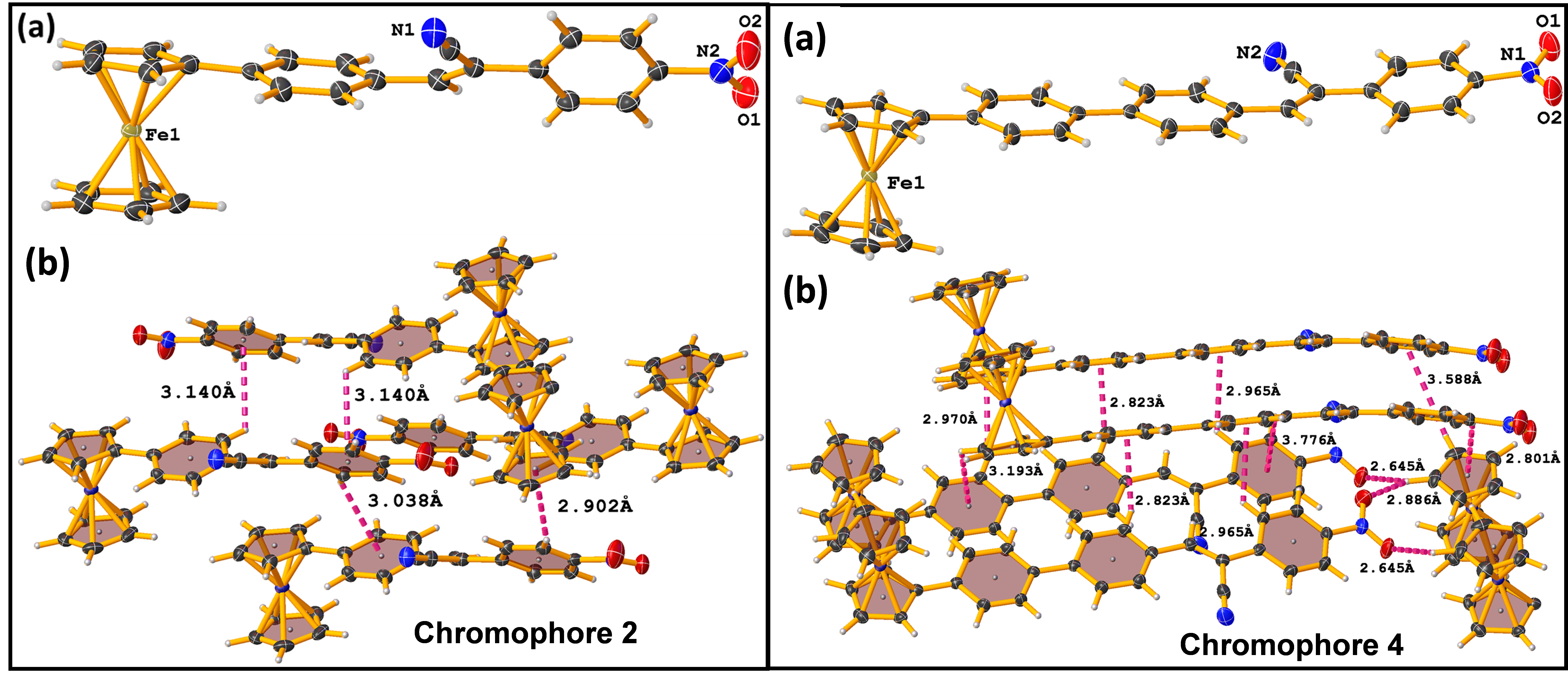
Fig.1. (a) Crystal structures and (b) non-covalent interactions for 2 and 4.
Table 1. Second-order nonlinear properties and computed HOMO, LUMO energy gap, dipole moment and second-order nonlinear optical parameters of pNA and chromophores 1-4.
S. NO
aSHG efficiency (mV)
bµβHRS (×10-30 esu)
cµgas/
µCHCl3
dβHRS (×10-30 esu)
eEHOMO (eV)
eELUMO (eV)
eEnergy gap
(eV)
bα0
(×10-24 esu)
fβ0
(×10-30 esu)
pNA (ref)
40
17.1
6.03/7.56
2.26
- 7.212
- 2.793
4.61
48.485
1.467
1
24
---
8.05/9.37
---
-5.960
-2.796
3.16
27.024
1.989
2
35
56.5
8.99/10.58
5.34
-5.847
-3.187
2.67
60.579
2.432
3
33
---
9.26/10.67
---
-5.723
2.916
2.76
38.668
2.735
4
43
81.5
10.59/10.83
7.53
-5.675
-3.222
2.45
75.809
6.605
aSecond order nonlinear optical efficiency by Kurtz and Perry powder method. bIn anhydrous CHCl3 estimated at 10−5 M concentration HRS measurement values. cComputed dipole moment values in gas and CHCl3 solvent phase using B3LYP/6-31+G** level of theory and dβHRS was calculated using the computed μCHCl3 value from DFT calculations. eCalculated HOMO, LUMO, energy gap and fPolarizability (α0) and Hyperpolarizability (β0) using B3LYP/6-31+G** level of theory.
How to Cite
Article Details
Ferrocene, Nonlinear optics, HRS technique, Linear D-π-A Chromophore, AIE, DFT/TD-DFT calculations
[2] T. Viswanathan, M. Gopalakrishnan, K. Thirumoorthy, M. Prakash and N. Palanisami, J. Phys. Chem. C, 125, 8732−8740 (2021). https://pubs.acs.org/10.1021/acs.jpcc.0c11242.
[3] E. David, A. Colombo, C. Dragonetti and N. Palanisami, Chem. - A Eur. J., 2021, 27, 7124–7137. https://doi.org/10.1002/chem.202005059.
